Haemokinesis, Monash partner for laser blood incubator
Jessica Guttridge
Researchers have discovered a new approach to speed up the process of identifying blood from minutes to seconds

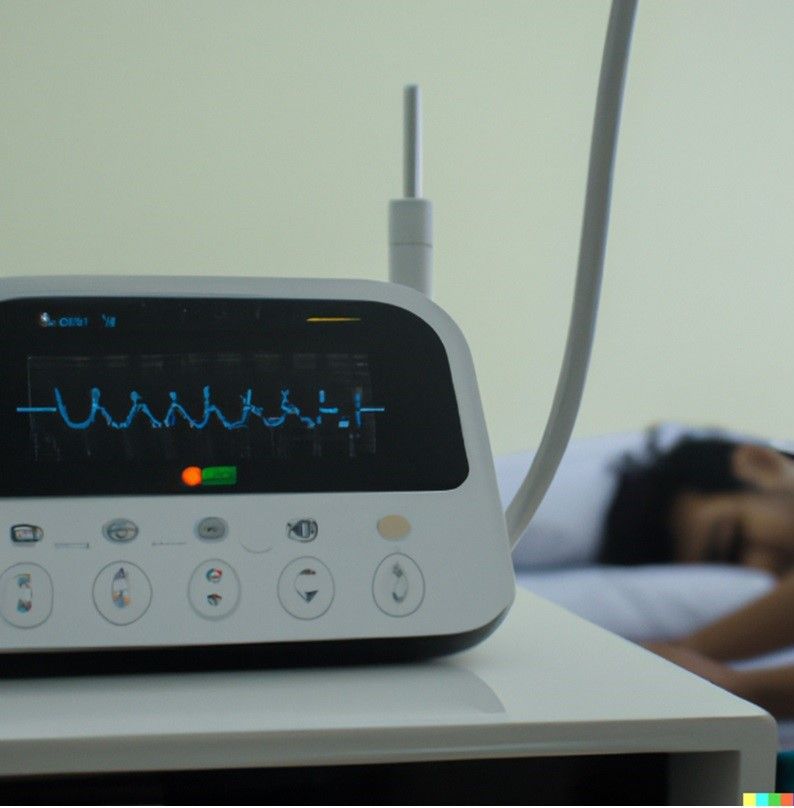
Monash University researchers, along with industry partner Haemokinesis, have developed the world’s first blood incubator using laser technology.
Blood transfusions require matching recipients, and screening the blood beforehand using traditional methods can take a quarter of an hour.
The laser incubation technology reduces the delay before a blood transfusion from 15 minutes to 40 seconds, which substantially affects a patient’s chance of survival.
“Giving blood transfusions to people isn’t as simple as giving O-negative to anybody. The ‘universal donor’ of O-negative blood can seriously harm a lot of people, even kill them,” explains Dr Clare Manderson, a researcher from the Bioresource Processing Institute of Australia (BioPRIA), based at Monash University.
The current industry gold standard is five minutes.
The study shows that laser incubation can improve pre-transfusion testing for millions of patients undergoing blood transfusions across the world, especially those having major surgery, women in labour or casualties of mass trauma who need fast blood transfusions.
Additionally, the shorter turnaround time saves on pathology costs, according to Haemokinesis.
Dr Clare Manderson discussed how the incubator works with Innovation Intelligence.
“The laser-incubator heats the blood-antibody sample to 37°C in under 30 seconds and keeps it at that temperature plus or minus one degree, for however long it needs to be. The gel is removed and put into a centrifuge where it is spun, separated and the blood and anti-body sample pass through the gel.
“The results are indicated by the position of the red blood cells in the gel. In positive blood groups the red blood cells sit on top of the gel, in negative blood groups the red blood cells pass through the gel.
“In our paper we looked at lots of different incubation times and we’re able to show that 40 seconds is enough to get positive results,” Dr Manderson said.
With the blood laser incubator, no significant damage was detected to the cells or antibodies while maintaining the temperature up to this time.
Vaughn Davies, head of sales at Haemokinesis, adds that the solution also creates a digital archive of the results which can be referred to if necessary.
“Traditionally laboratories have used the test tube system, where there are no permanently recorded results. You drop your reagent in the test tube, put the patient’s blood cells in, spin it in the centrifuge for a minute, read your results, then throw the tubes away.
“If there is any doubt or any question about it, how do you know what the result was?” Davies said.
While the technology isn’t yet commercially available in Australia, Haemokinesis holds a patent for this innovation as well as a Therapeutic Goods Administration (TGA) licence and is in discussions with hospitals and pathology companies.
Haemokinesis is already selling products
to distributors across South East Asia, and Europe.
The company also has commercialised a separate blood diagnostics tool called GLIF
or in length: Group Legible Immunohaematology Format, which is a blood test that can be conducted by untrained individuals and performed out of curiosity.
“The world of pre-transfusion of blood group typing is huge, and it’s really important that it’s done quickly and accurately to help save lives,” Dr Manderson said.

In 2016 I published a blog article titled Moonshots for Australia: 7 For Now. It’s one of many I have posted on business and innovation in Australia. In that book, I highlighted a number of Industries of the Future among a number of proposed Moonshots. I self-published a book, Innovation in Australia – Creating prosperity for future generations, in 2019, with a follow-up COVID edition in 2020. There is no doubt COVID is causing massive disruption. Prior to COVID, there was little conversation about National Sovereignty or supply chains. Even now, these topics are fading, and we remain preoccupied with productivity and jobs! My motivation for this writing has been the absence of a coherent narrative for Australia’s business future. Over the past six years, little has changed. The Australian ‘psyche’ regarding our political and business systems is programmed to avoid taking a long-term perspective. The short-term nature of Government (3 to 4-year terms), the short-term horizon of the business system (driven by shareholder value), the media culture (infotainment and ‘gotcha’ games), the general Australian population’s cynical perspective and a preoccupation with a lifestyle all create a malaise of strategic thinking and conversation. Ultimately, it leads to a leadership vacuum at all levels. In recent years we have seen the leadership of some of our significant institutions failing to live up to the most basic standards, with Royal Commissions, Inquiries and investigations consuming excessive time and resources. · Catholic Church and other religious bodies · Trade Unions · Banks (and businesses generally, take casinos, for example) · the Australian Defence Force · the Australian cricket teams · our elected representatives and the staff of Parliament House As they say, “A fish rots from the head!” At best, the leadership behaviour in those institutions could be described as unethical and, at worst….just bankrupt! In the last decade, politicians have led us through a game of “leadership by musical chairs” – although, for now, it has stabilised. However, there is still an absence of a coherent narrative about business and wealth creation. It is a challenge. One attempt to provide such a narrative has been the Intergenerational Reports produced by our federal Government every few years since 2002. The shortcomings of the latest Intergenerational Report Each Intergenerational Report examines the long-term sustainability of current government policies and how demographic, technological, and other structural trends may affect the economy and the budget over the next 40 years. The fifth and most recent Intergenerational Report released in 2021 (preceded by Reports in 2002, 2007, 2010 and 2015) provides a narrative about Australia’s future – in essence, it is an extension of the status quo. The Report also highlights three key insights: 1. First, our population is growing slower and ageing faster than expected. 2. The Australian economy will continue to grow, but slower than previously thought. 3. While Australia’s debt is sustainable and low by international standards, the ageing of our population will pressure revenue and expenditure. However, its release came and went with a whimper. The recent Summit on (what was it, Jobs and Skills and productivity?) also seems to have made the difference of a ‘snowflake’ in hell in terms of identifying our long-term challenges and growth industries. Let’s look back to see how we got here and what we can learn. Australia over the last 40 years During Australia’s last period of significant economic reform (the late 1980s and early 1990s), there was a positive attempt at building an inclusive national narrative between Government and business. Multiple documents were published, including: · Australia Reconstructed (1987) – ACTU · Enterprise Bargaining a Better Way of Working (1989) – Business Council of Australia · Innovation in Australia (1991) – Boston Consulting Group · Australia 2010: Creating the Future Australia (1993) – Business Council of Australia · and others. There were workshops, consultations with industry leaders, and conferences across industries to pursue a national microeconomic reform agenda. Remember these concepts? · global competitiveness · benchmarking · best practice · award restructuring and enterprising bargaining · training, management education and multiskilling. This agenda was at the heart of the business conversation. During that time, the Government encouraged high levels of engagement with stakeholders. As a result, I worked with a small group of training professionals to contribute to the debate. Our contribution included events and publications over several years, including What Dawkins, Kelty and Howard All Agree On – Human Resources Strategies for Our Nation (published by the Australian Institute of Training and Development). Unfortunately, these long-term strategic discussions are nowhere near as prevalent among Government and industry today. The 1980s and 1990s were a time of radical change in Australia. It included: · floating the $A · deregulation · award restructuring · lowering/abolishing tariffs · Corporatisation and Commercialisation Ross Garnaut posits that the reforms enabled Australia to lead the developed world in productivity growth – given that it had spent most of the 20th century at the bottom of the developed country league table. However, in his work, The Great Reset, Garnaut says that over the next 20 years, our growth was attributable to the China mining boom, and from there, we settled into “The DOG days” – Australia moved to the back of a slow-moving pack! One unintended consequence of opening our economy to the world is the emasculation of the Australian manufacturing base. The manic pursuit of increased efficiency, lower costs, and shareholder value meant much of the labour-intensive work was outsourced. Manufacturing is now less than 6% of our GDP , less than half of what it was 30 years ago!