Device Technologies: Bringing ANZ the best MedTech for 30 years
Partnership and innovation for continued success in the Australian healthcare industry and beyond.
Device Technologies has been a true pioneer in the Australian healthcare and medical technology industries.
Device has been at the forefront of bringing the most innovative technologies into ANZ, the flagship being da Vinci® Robotic Surgery. Its 30-year history of accolades include an instrumental role in the establishment of the RPA Surgical and Robotic Training Institute in Sydney, the only one of its kind in the southern hemisphere.
The company has re-defined the role of cutting-edge medical technology supply to include intensive product evaluation and recommendation to hospitals and specialists. Services and expertise extend to clinical education and training as well as managing regulatory requirements. Device has thus evolved to become a vital knowledge centre within the largest employing industry in Australia.
Founders’ vision to source the best worldwide
It all began in 1992 when four friends started operations out of a small garage in Seaforth, yet with a vision to distribute across ANZ the best medical devices from around the world. John McQuillan, Peter Ord, Kevin Ryan and Bill Walker had been working for large American companies in the 1980s and though they’d enjoyed a level of success, their shared goal was to provide medical specialists and hospitals access to first-class medical technologies from all parts of the globe.
This vision has carried on over three decades. Today, the Device Business Development team continues to partner with world-leading manufacturers, while developing new relationships with innovative MedTech suppliers globally.
Building long-lasting relationships with suppliers, hospitals and surgeons is cornerstone to the company’s operations, wherein the passion and tenure of staff organisation-wide remains key.
A 360-degree approach
This has developed into a symbiotic relationship with Device’s dynamic network of surgeons and healthcare executives, who are constantly interested in emerging and innovative technologies and procedures to ultimately enhance patient outcomes.
After a new product or solution has been identified, a rigorous assessment involving due diligence, health economics studies and registration with the Federal Government’s Therapeutic Goods Administration (TGA) is conducted. Promotion, clinical education and training, and end-user feedback are subsequently undertaken. This pathway harnesses the skills and experience of a team comprising healthcare professionals, in-house product specialists, clinical educators, engineers and regulatory affairs officers.
CEO Michael Trevaskis remarks, “Few Australians fully realise the sheer calibre of doctors and surgeons in this country. They are at the forefront of what is on the global medical technology landscape and it remains our privilege to work closely with them. We’re also proud of the complementary role we play advising on redesign of hospital infrastructure and procedures.”
Pioneering Robotic Surgery
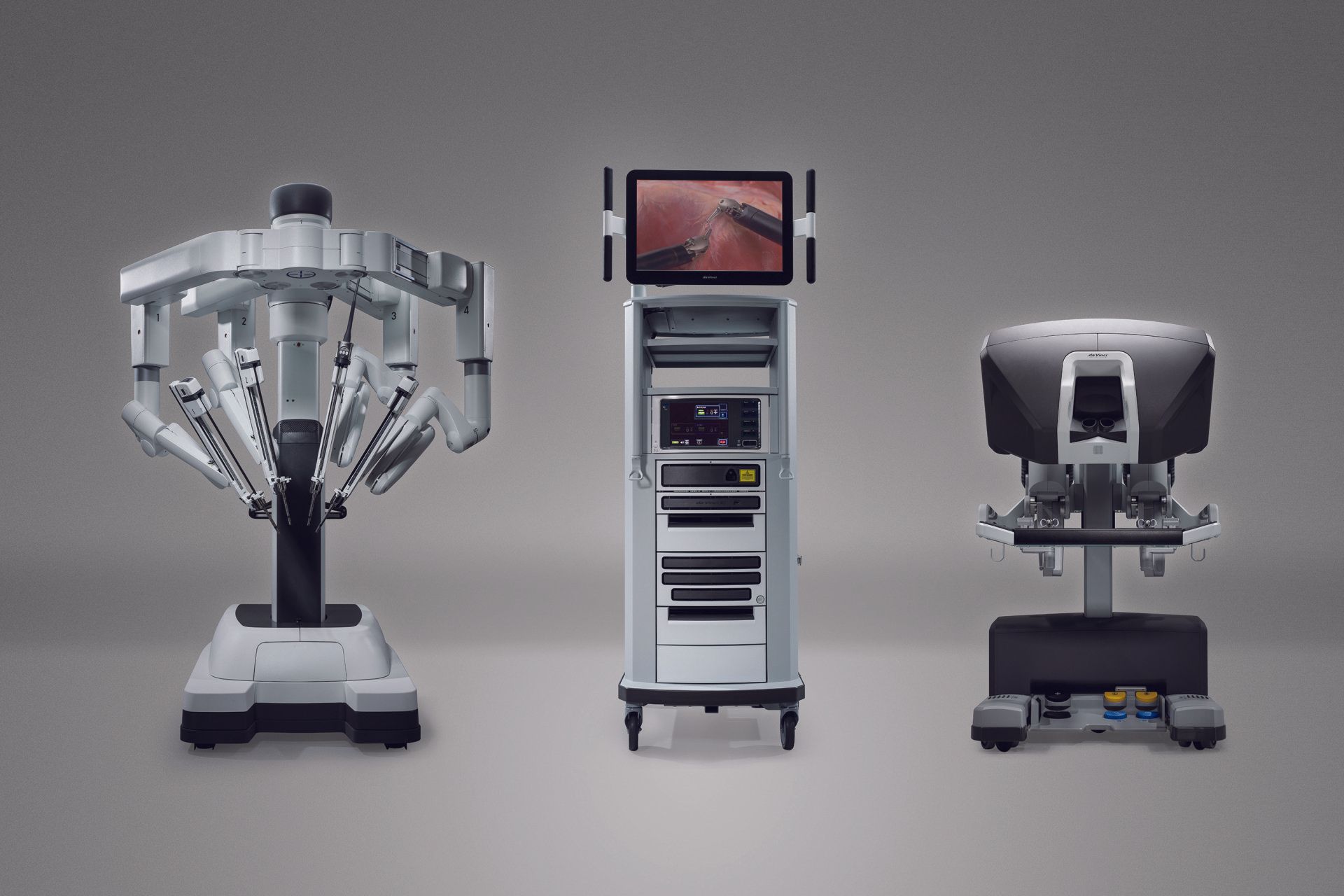
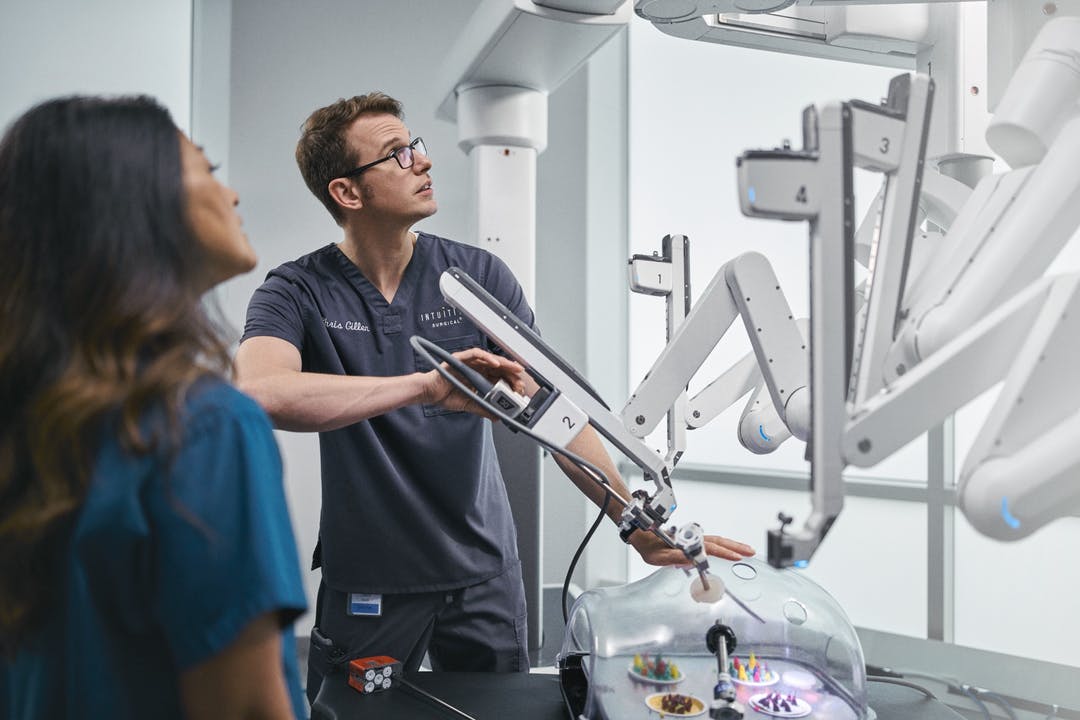
The da Vinci robot is a state-of-the-art surgical robot, manufactured by California based company Intuitive. The partnership between Device and Intuitive since 2003 has been paramount to the integration of da Vinci platforms in ANZ, pioneering new capabilities in the operating room. This technology enables surgeons to perform minimally invasive surgery resulting in significantly improved patient outcomes. At present, 80 da Vinci Surgical Systems are in operation in ANZ.
This hasn’t been Device’s only national first. When total artificial hearts and TAVIs were at the forefront in valve replacement and heart surgery, Device played a pivotal role in their introduction to Australian cardiac surgeons and cardiologists. In addition, Device has played a critical role in enhancing infection control in healthcare, partnering closely with customers. In more recent times, Device Technologies has worked closely with governments to ensure access to world-leading ventilators, intensive care supplies, PPE, rapid antigen testing kits and cleaning chemistries in support of the fight against COVID-19.
Innovative Business Development
New business development opportunities are being identified, explored and materialised by Device on a continual basis. States Michael, “In the 17 smaller companies we’ve so far welcomed to the Device Group, we’ve gained some of the best innovators and the best innovations and integrated them into our business.
Through these acquisitions and partnerships, Device has diversified into areas such as Aged Care, Veterinary, and local manufacturing, including 3D printing. This capability, which will be vitally important to Australia in the coming years and decades, gives Device global credibility and differentiates us even further from multi-national subsidiaries.”
Employment opportunities and a culture of fun
Like most companies, talent management is an important part of the Device business philosophy. In fact, their graduate programs have an industry-leading rate of conversion from paid internships to permanent roles. For the last eight years, the Regulatory and Quality Assurance team has run an active graduate program. To date, 12 interns have completed the program, with six moving on to permanent employment.
From the start in 1992, the goal has been to employ professional people and provide an environment in which they can grow and thrive. Says Michael, “The beauty of Device is that people can have fun, while also making a meaningful contribution. When people can be successful, feel safe (both mentally and physically), have a voice, love the business and love MedTech, then you’ve created a culture of success.” The company’s recent expansion into Asia has seen these benefits mirrored for staff now working there.
Growth in Asia
Heeding a comparatively under-developed healthcare industry, expanding the business into Asia had been the natural next step for Device for some years. Asia staff numbers have grown to over 50 in two years to complement the 920 staff members in Australia and 55 in New Zealand. In two years, operations have been launched in Singapore, Thailand, Vietnam, Malaysia, and recently the Philippines, with a commitment to attracting, developing and investing in local talent and skills.
Beyond 30 years
With a rich history and continued momentum, Device Technologies is on course to engage in an exciting future envisioned. Beyond entrepreneurial accomplishment, Device displays how companies which interact effectively with the burgeoning technology sphere can produce collateral benefits – in this case medical, community health and social. When it comes to passion for the business and a love for MedTech, this team is certainly in no short supply.