Bench to Bedside
Translational research is key to improving the health of Australians. How can we improve it?

Translational research focuses on health and medical research which aims to translate laboratory-based research into the clinic to improve the detection, diagnosis and treatment of disease and improve health outcomes for the population. In each case, it almost invariably takes more than a decade and costs more than $1bn to take a novel scientific discovery through to a new diagnostic or treatment for patients. Despite this time and investment, many discoveries still fall by the wayside, even those that have reached pivotal phase III human clinical trials.
Over the past two decades, governments around the world have increasingly invested in programs to support translational research pathways and training opportunities that aim to decrease the time it takes for such discoveries to reach the clinic. In parallel, there has been rapid growth in the number of scientific journals focusing specifically on translational science, and likewise in conferences and courses for training scientists and clinicians in translational-research approaches. In Australia, the Commonwealth Government’s investment in the Medical Research Future Fund (MRFF) has demonstrated its focus on ensuring the investment in research not only generates new knowledge but that this knowledge ultimately leads to better health outcomes for Australians.
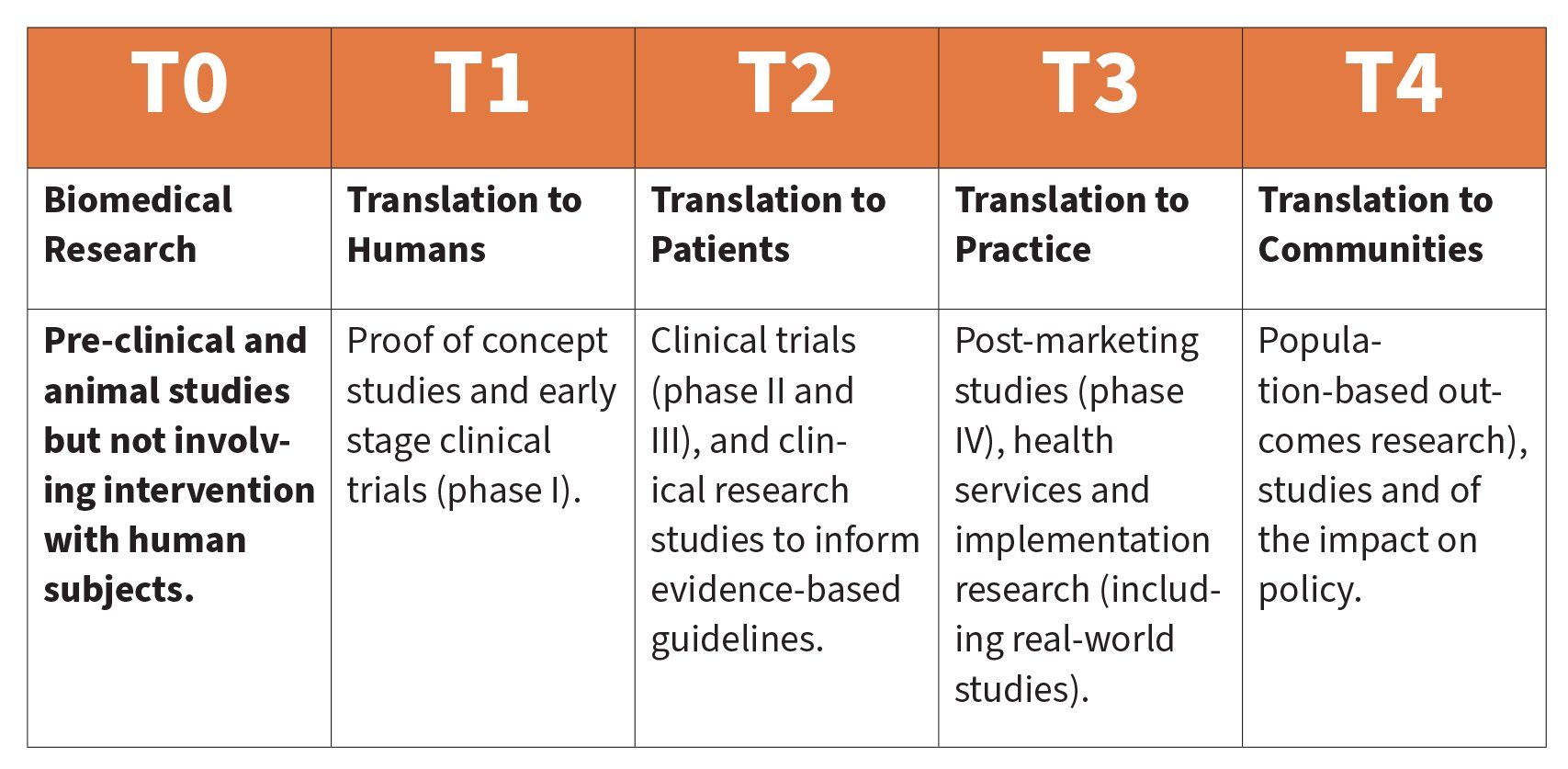
Stages of Translational Research
Translational research can be classified to describe where a specific project sits on the translational research continuum. Whilst this pathway can lead to health benefits for the broader population, ideally researchers, industry, clinicians (and their patients) and research funders are involved in all translational stages Broadly, there are two approaches to progression along the translational pathway. The first begins in the clinic, where a new diagnostic or therapy is applied to patients in a clinical setting, leading ultimately to evidence to support implementation to the patient population. The second approach is where a discovery leads to early-stage translation (T1), often in an academic research setting, followed by links with industry, which then takes on the role (usually in collaboration with researchers) to develop the next stage of the translational pathway (T2 and beyond). There are several key components to the translational research ecosystem, which include access to research collaborations and state-of-the-art research equipment; direct linkages to clinicians and their patients; and dedicated clinical-trial facilities.
In early October 2020, the Nobel Prize in Chemistry was awarded to the first all-female team, Professors Jennifer Doudna and Emanuelle Charpentier, for the discovery of gene editing using the platform of CRISPR/CAS9 technology. This scientific advance has revolutionised discovery research and could potentially translate into new therapies for many diseases.
CRISPR/CAS9 has provided researchers with novel and powerful tools to support improved understanding of genetics of diseases. It is supporting the development of precise cellular and animal models of human diseases. It is now feasible to generate in vivo animal models specific to diseases within weeks. Importantly, this technology also has tremendous therapeutic potential, particularly for developing gene therapies (where a patient-specific mutation is genetically corrected) for human diseases that are currently inadequately treated. Already there are clinical trials being undertaken using ex vivo gene therapy on patients with a number of blood disorders, including severe combined immunodeficiency, Fanconi anaemia and sickle cell disease.
The discovery of the CRISPR/CAS9 and its ultimate application in the clinic is a compelling example of the importance of bridging discovery science and translational research pathways: bench to bedside to bench.
During the COVID-19 pandemic, it became clear that the limited MedTech manufacturing industry in Australia contributed to critical supply-chain gaps and delays. In Australia, we excel in the academic sector (universities and medical research Institutes) for research discoveries and early-stage translation. However, there is a growing realisation of the need to develop the MedTech manufacturing capability nationally. Supporting the MedTech industry through emerging startup and SME companies to develop their translational pathways locally is now seen as critically important for the future of both translational research development and advanced manufacturing.
There are challenges to supporting the efficient progression of developing evidence along the translational pathway. These include the need to link translational-research developments back to their origin (discovery science) so we ensure there is an ongoing pathway of new diagnostics and potential treatments flowing through to basic discovery laboratories and preclinical studies. This also requires ongoing financial investment through traditional funding sources such as the National Health and Medical Research Council (NHMRC) and the Australian Research Council (ARC).
Another challenge is to support clinician scientists to ensure translational research pathways are clinically relevant. There is a need to maintain clinical connectedness between the discovery scientists to ensure that the key questions in the clinic today are being addressed in the basic science laboratories (bench to bedside and back to the bench). The important final challenge is to make clinical-trial platforms readily available to support translational research nationally.
Professor Scott Bell is CEO for the Translational Research Institute.
This article is taken from the recently published digital book
Australia's Nobel Laureates Vol III State of our Innovation Nation: 2021 and Beyond